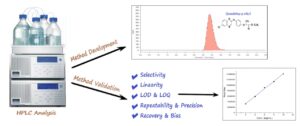
 A novel quantitative method for the determination of quizalofop-p-ethyl herbicide has been developed and validated using high-performance liquid chromatography with ultraviolet detection (HPLC-UV), which is cost-effective, easy to use, and rapid. This active ingredient is widely used as a systemic herbicide belonging to the aryloxy phenoxy-propionates group and is the active component in Torgy 5% EC® product, a formulation specifically designed for controlling unwanted weeds in various crops. The mobile phase is composed of a gradient of miscible solvents (acetonitrile and distilled water) in the 4:1 (v/v) ratio at a flow rate of 1.5 ml/min with UV detection at 260 nm. The reversed-phase brownlee™ C-18 column that was thermostated at 30°C used for quantification. The method’s validation procedures, such as selectivity, linearity, range, LOD, LOQ, and trueness (bias and recovery), were discussed according to ICH guidance standards. The validation results demonstrated that this innovative method is suitable for assessing the content of quizalofop-p-ethyl within the 2–10 mg/mL range. The high value of the regression coefficient (R²=0.9971) underscores that the method is fit-for use for analytical applications.
A novel quantitative method for the determination of quizalofop-p-ethyl herbicide has been developed and validated using high-performance liquid chromatography with ultraviolet detection (HPLC-UV), which is cost-effective, easy to use, and rapid. This active ingredient is widely used as a systemic herbicide belonging to the aryloxy phenoxy-propionates group and is the active component in Torgy 5% EC® product, a formulation specifically designed for controlling unwanted weeds in various crops. The mobile phase is composed of a gradient of miscible solvents (acetonitrile and distilled water) in the 4:1 (v/v) ratio at a flow rate of 1.5 ml/min with UV detection at 260 nm. The reversed-phase brownlee™ C-18 column that was thermostated at 30°C used for quantification. The method’s validation procedures, such as selectivity, linearity, range, LOD, LOQ, and trueness (bias and recovery), were discussed according to ICH guidance standards. The validation results demonstrated that this innovative method is suitable for assessing the content of quizalofop-p-ethyl within the 2–10 mg/mL range. The high value of the regression coefficient (R²=0.9971) underscores that the method is fit-for use for analytical applications.
quizalofop-p-ethyl, HPLC, method validation, LOD, LOQ, linearity
