1 Pharmaceutical Sciences Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran
2 Department of Medicinal Chemistry, School of Pharmacy and Pharmaceutical Sciences Research Center, Mazandaran University of Medical Sciences, Sari, Iran
3 Department of Environmental Health, Faculty of Health, Mazandaran University of Medical Sciences, Sari, Iran
* Correspondence to: Chemistry_2021@yahoo.com; MA.Ebrahimzadeh@mazums.ac.ir
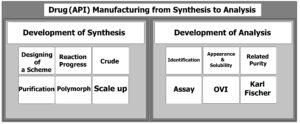 Simply, production of a drug needs two main steps; the first step is the chemical synthesis, and the second one is the pharmaceutical analysis. At the first step, the desired drug must be synthesized via the lowest possible production cost. The synthesis scheme must be designed to proceed the reaction with the highest yield, cheapest solvents, and mildest possible conditions. It should also be user friendly (for operators of the production unit), as fast as possible, with lower steps leading to a highly pure active pharmaceutical ingredient (API). The pharmaceutical analysis step contains a series of the main analytical tests such as instrumental (HPLC, GC, XRD, and …), as well as physicochemical ones (like solubility, ash, heavy metals, …). These tests determine the quality of an API for being applied in the finish line formulation of a drug.
Simply, production of a drug needs two main steps; the first step is the chemical synthesis, and the second one is the pharmaceutical analysis. At the first step, the desired drug must be synthesized via the lowest possible production cost. The synthesis scheme must be designed to proceed the reaction with the highest yield, cheapest solvents, and mildest possible conditions. It should also be user friendly (for operators of the production unit), as fast as possible, with lower steps leading to a highly pure active pharmaceutical ingredient (API). The pharmaceutical analysis step contains a series of the main analytical tests such as instrumental (HPLC, GC, XRD, and …), as well as physicochemical ones (like solubility, ash, heavy metals, …). These tests determine the quality of an API for being applied in the finish line formulation of a drug.
In the present paper, we have simply described the two main steps for manufacturing a drug API which contains the synthesis step as well as the pharmaceutical analytical part. Also, some key notes for drug synthesis step will be presented.
drug synthesis, pharmaceutical analysis, drug manufacturing, pharmaceutical industry, API