1 Department of Chemistry, Federal University of Petroleum Resources, Effurun, Nigeria
2 Department of Chemistry, Joseph Sarwuan Tarkaa University, Makurdi, Nigeria
* Correspondence to: uzah2t@gmail.com
Abstract
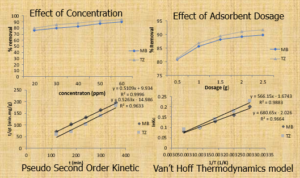 This research work assessed the adsorption performance of silica xerogels for the removal of a binary solution of methylene blue and tartrazine dyes. Silica xerogel was extracted from rice husk using the gel method and modified with 1.0 M HNO3 (nitric acid). Using batch adsorption methods, the effect of initial concentration, temperature, ionic strength, contact time, and adsorbent dosage on the adsorption process of methylene blue and tartrazine was studied using binary solutions of the dyes. The percentage removal of the dyes studied increased as the initial concentration was varied from 20 ppm to 60 ppm. The removal efficiency decreased as the temperature was varied from 30 0C to 50 0C. The adsorption efficiency showed an increase at higher pH for methylene blue and at lower pH for tartrazine due to their ionic nature. The variation of contact time between 2 and 6 hours showed a sharp increase in dye removal from 2 to 5 hours, but a slow increase after 5 hours. The percentage removal of dyes increased as the adsorbent dosage increased between 0.5 to 2.5 g. The effect of ionic strength on adsorption efficiency was found to decrease as the concentration of NaCl was varied from 0.02 to 0.10 M. The experimental data were tested using the adsorption isotherms models of Langmuir, Freundlich, and Temkin. The Langmuir model was found to be the best fit among the three isotherms used for the analysis, as all the R2 and RL values favoured the isotherm. The data showed that the adsorption process can best be described by the pseudo-second order kinetics model, as the R2 values for all the dyes are more than those of the pseudo-first order model. Therefore, it can be concluded that the silica xerogel is a potential adsorbent for the uptake of pollutants in wastewater.
This research work assessed the adsorption performance of silica xerogels for the removal of a binary solution of methylene blue and tartrazine dyes. Silica xerogel was extracted from rice husk using the gel method and modified with 1.0 M HNO3 (nitric acid). Using batch adsorption methods, the effect of initial concentration, temperature, ionic strength, contact time, and adsorbent dosage on the adsorption process of methylene blue and tartrazine was studied using binary solutions of the dyes. The percentage removal of the dyes studied increased as the initial concentration was varied from 20 ppm to 60 ppm. The removal efficiency decreased as the temperature was varied from 30 0C to 50 0C. The adsorption efficiency showed an increase at higher pH for methylene blue and at lower pH for tartrazine due to their ionic nature. The variation of contact time between 2 and 6 hours showed a sharp increase in dye removal from 2 to 5 hours, but a slow increase after 5 hours. The percentage removal of dyes increased as the adsorbent dosage increased between 0.5 to 2.5 g. The effect of ionic strength on adsorption efficiency was found to decrease as the concentration of NaCl was varied from 0.02 to 0.10 M. The experimental data were tested using the adsorption isotherms models of Langmuir, Freundlich, and Temkin. The Langmuir model was found to be the best fit among the three isotherms used for the analysis, as all the R2 and RL values favoured the isotherm. The data showed that the adsorption process can best be described by the pseudo-second order kinetics model, as the R2 values for all the dyes are more than those of the pseudo-first order model. Therefore, it can be concluded that the silica xerogel is a potential adsorbent for the uptake of pollutants in wastewater.
Adsorption, Methylene blue, Tartrazine, Silica Xerogel, Adsorbent, Rice Husk
