2025 vol 4 issue 1
Full paper
Abdelhak Ouled Aitouna 1,2, Bouchra Rossafi 1, Habib El Alaoui El Abdallaoui 1, Abdellah Zeroual 1 *
1 Molecular Modelling and Spectroscopy Research Team, Faculty of Science, Chouaïb Doukkali University, P.O. Box 20, 24000 El Jadida, Morocco
2 Laboratory of Biomolecular Chemistry, Natural Substances and Reactivity, URAC 16, Faculty of Sciences Semlalia, Cadi Ayyad University, P.O. Box 2390, Marrakech, Morocco
* Correspondence to: zeroualabdellah2@gmail.com
pp. 18-28
DOI: 10.58332/scirad2025v4i1a02
Abstract
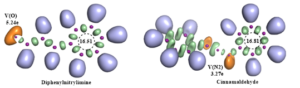 The theoretical study of the reaction between cinnamaldehyde and nitrile-imine, conducted using the MEDT/B3LYP/6-311G(d,p) method, clarified the nucleophilic and electrophilic roles of the two components, demonstrating that the dipole acts as a nucleophile, while the dipolarophile acts as an electrophile. Parr functions indicate that the double bond of the dipolarophile is identified as the most reactive site, providing a clear explanation for the chemoselectivity observed in the experiment. Furthermore, the study of interactions reveals that the formation of the first bond involves the most electrophilic carbon. The analysis of the products and transition states reveals that one of the products is thermodynamically favored, while a certain transition state represents the dominant kinetic pathway.
The theoretical study of the reaction between cinnamaldehyde and nitrile-imine, conducted using the MEDT/B3LYP/6-311G(d,p) method, clarified the nucleophilic and electrophilic roles of the two components, demonstrating that the dipole acts as a nucleophile, while the dipolarophile acts as an electrophile. Parr functions indicate that the double bond of the dipolarophile is identified as the most reactive site, providing a clear explanation for the chemoselectivity observed in the experiment. Furthermore, the study of interactions reveals that the formation of the first bond involves the most electrophilic carbon. The analysis of the products and transition states reveals that one of the products is thermodynamically favored, while a certain transition state represents the dominant kinetic pathway.
Keywords
cinnamaldehyde, chemoselectivity, MEDT, B3LYP/6-311G(d,p)
First published: 13.02.2025
